Overview
Our laboratory seeks to understand how molecules encode intermolecular interactions, how those interactions give rise to supramolecular organization, and the properties that emerge from that organization. We are particularly interested in the ordering of molecules at interfaces, and associated interfacial properties. In some instances, the interfaces are macroscopic, such as the surface of a polymeric film. In other cases, the surfaces are buried and nanoscopic, such as interfaces defined by the self-assembly of surfactant molecules into micelles.
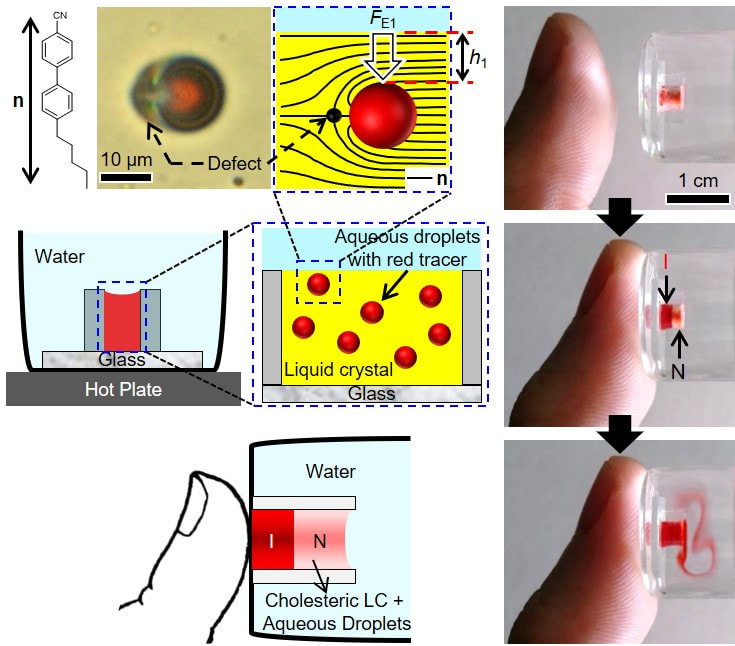
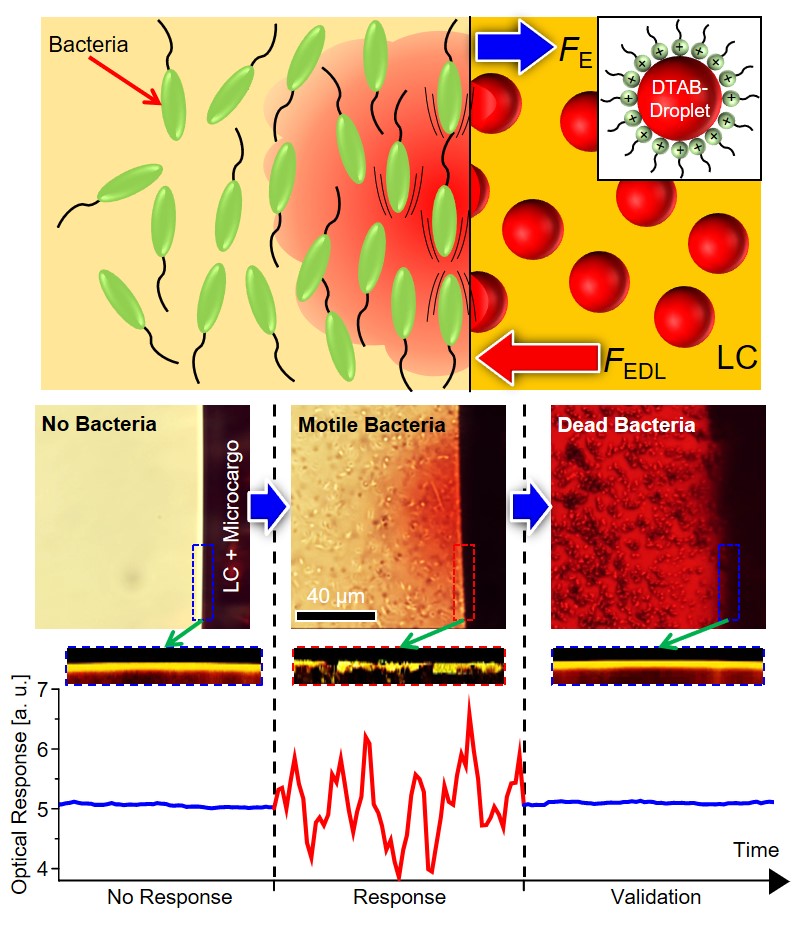
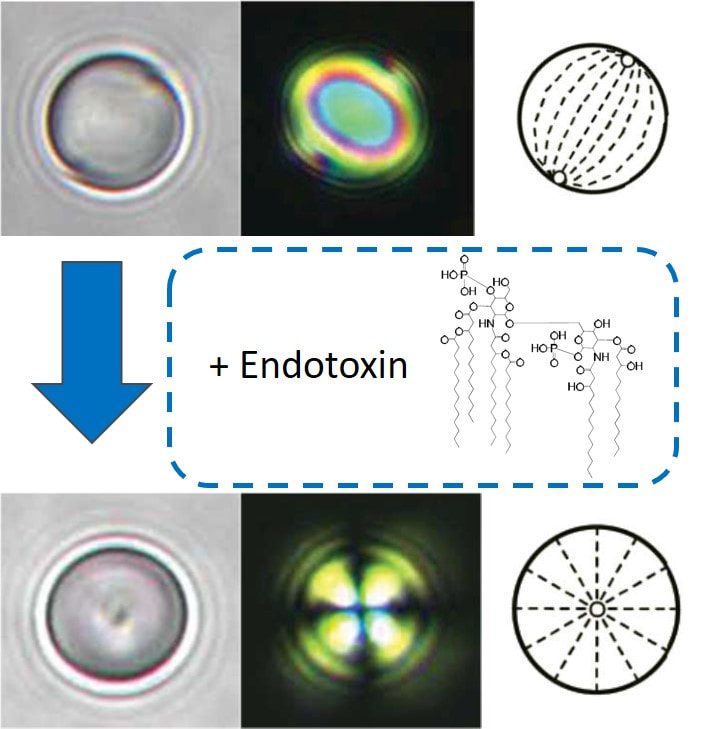
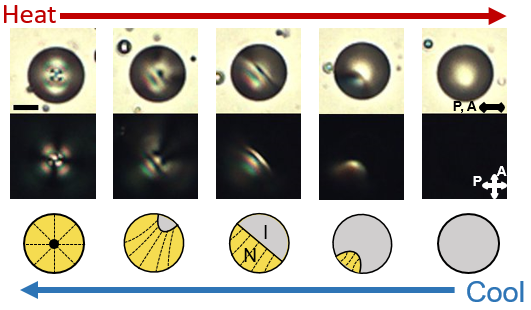
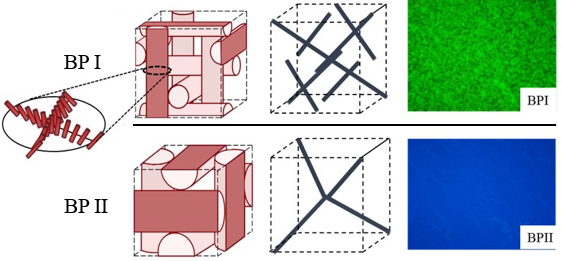
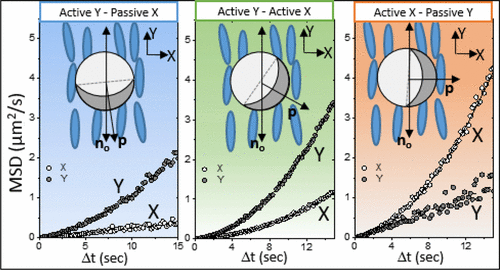
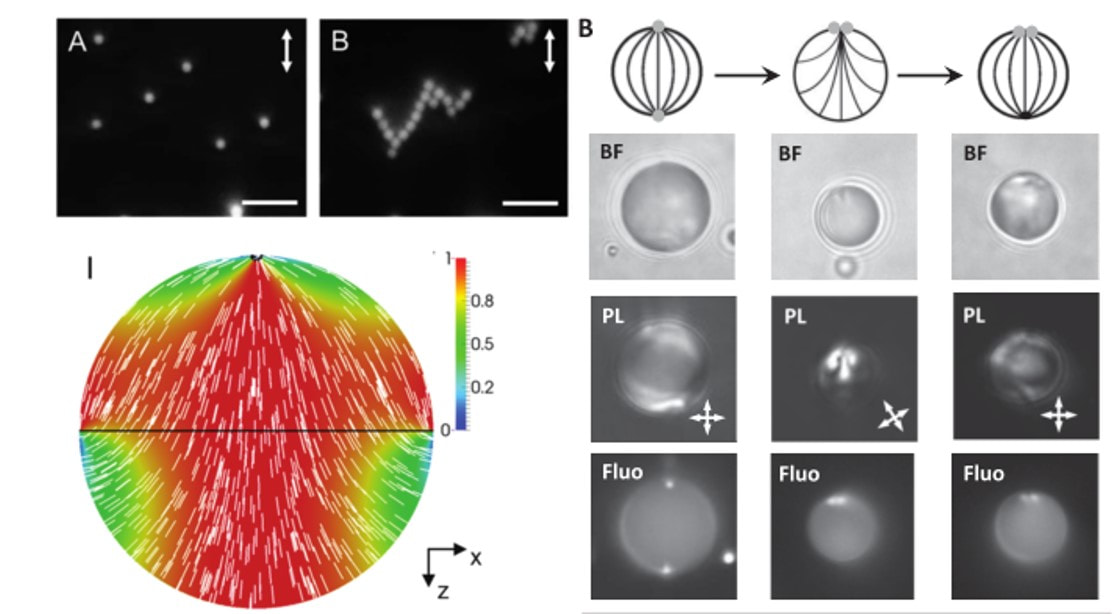
The molecular materials that we study are held together by non-covalent interactions, and thus they tend to be soft and readily reorganized by external stimuli. For example, we are exploring the properties of liquid crystals. As the name suggests, molecules within liquid crystals possess the mobility of liquids yet exhibit the long-range order typical of crystals. We are studying the ordering of liquid crystals at interfaces, and how chemical, physical and biomolecular interactions can trigger changes in the ordering of liquid crystals.
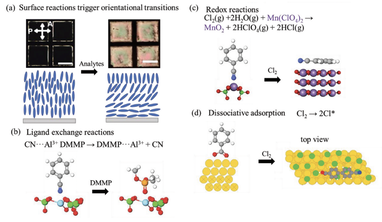
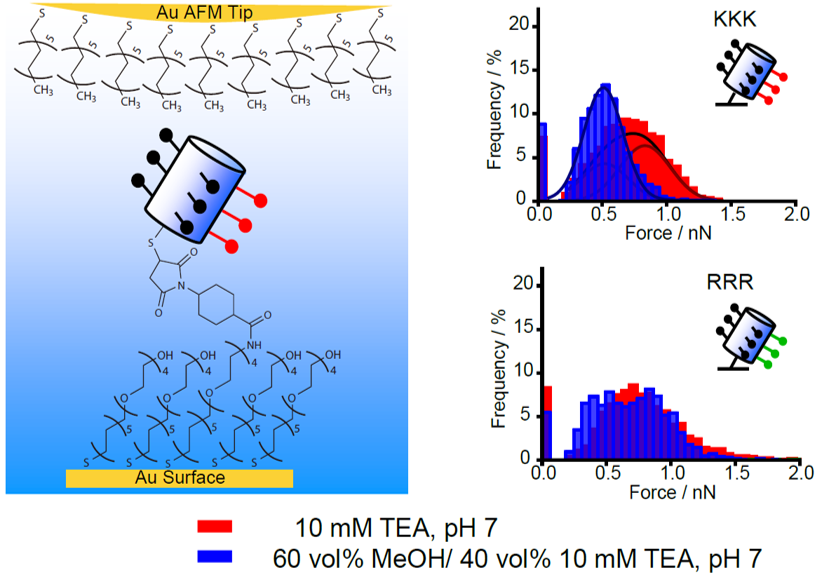
We are also investigating the ordering of water at interfaces, with a focus on understanding how nanoscopic chemical patterns lead to interactions between molecules dispersed in water. This research is providing new design rules for hydrophobically-driven self-assembly.
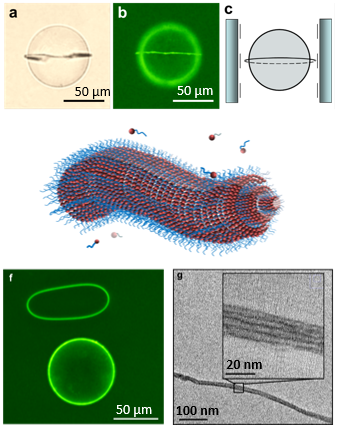
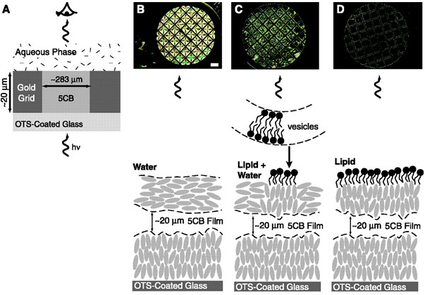
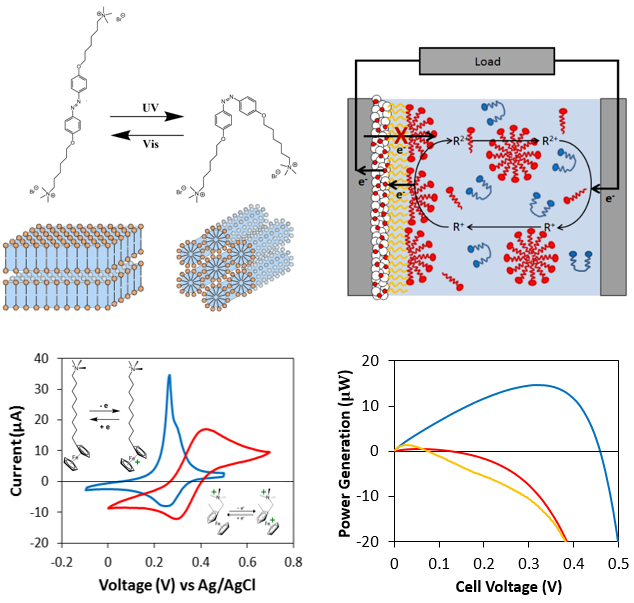
A third area that we are studying involves molecular self-assembly of amphiphilic molecules. We are designing surfactants that incorporate redox-active and light-sensitive groups and exploring how changes in the states of these molecules influence their self-assembly in solution and at interfaces. We are also exploring how topological defects formed in liquid crystals can serve as virtual templates for the self-assembly of amphiphilic molecules.
Our research projects typically require collaboration, as we address challenges that benefit from multiple perspectives and approaches. We work closely, in particular, with collaborators with expertise in electronic structure calculations, molecular simulations and meso-scale modeling. In some cases, theory is used to interpret experimental observations and, in other instances, it serves to guide experiments. We also collaborate with researchers from chemistry, physics, biochemistry, microbiology, applied mathematics, pharmacy, veterinary medicine and medicine.
The molecular materials that we study are held together by non-covalent interactions, and thus they tend to be soft and readily reorganized by external stimuli. For example, we are exploring the properties of liquid crystals. As the name suggests, molecules within liquid crystals possess the mobility of liquids yet exhibit the long-range order typical of crystals. We are studying the ordering of liquid crystals at interfaces, and how chemical, physical and biomolecular interactions can trigger changes in the ordering of liquid crystals.
We are also investigating the ordering of water at interfaces, with a focus on understanding how nanoscopic chemical patterns lead to interactions between molecules dispersed in water. This research is providing new design rules for hydrophobically-driven self-assembly.
A third area that we are studying involves molecular self-assembly of amphiphilic molecules. We are designing surfactants that incorporate redox-active and light-sensitive groups and exploring how changes in the states of these molecules influence their self-assembly in solution and at interfaces. We are also exploring how topological defects formed in liquid crystals can serve as virtual templates for the self-assembly of amphiphilic molecules.
Our research projects typically require collaboration, as we address challenges that benefit from multiple perspectives and approaches. We work closely, in particular, with collaborators with expertise in electronic structure calculations, molecular simulations and meso-scale modeling. In some cases, theory is used to interpret experimental observations and, in other instances, it serves to guide experiments. We also collaborate with researchers from chemistry, physics, biochemistry, microbiology, applied mathematics, pharmacy, veterinary medicine and medicine.